Infectious diseases have always posed a threat to human health. But perhaps never more than today? “That is a difficult question to answer. But there is a whole range of risk factors that favour infections with previously unknown pathogens and make them more likely to spread today,“ says Prof Fabian Leendertz, the founding director of the HIOH. “Increasing human encroachment into wildlife habitats, loss of biodiversity, ongoing climate change and globalisation all play an important role here.“

Leap from animals to humans
The transmission of pathogens from animals to humans is called zoonosis, and unfortunately, we have recently seen some pertinent examples of this: Like the SARS-CoV-2 virus, the pathogen of COVID-19, which presumably leaped to humans via a bat. And the MPXV virus (monkeypox virus), the causative agent of monkeypox, whose outbreak was recently declared an emergency of international concern by the World Health Organisation. “Zoonoses are always volatile and can have far-reaching consequences. In fact, most of the infectious diseases we deal with today have a zoonotic origin,“ Leendertz explains. For example, one of the oldest known zoonoses is the transmission of the measles virus from cattle to humans around 500 BC, as suggested by new genetic mutation analyses of measles viruses. Once a pathogen has found a new host, i.e. humans, that ensures its further spread, it is usually there to stay. “Sometimes it takes only a single transmission event for this to happen. In the worst case, an infection scenario can develop so rapidly due to globalisation that it can become a world-wide pandemic. Which is what we have seen in the last 2.5 years with the Corona pandemic.“
Because he has always had a particular interest in the diseases of wild animals, Leendertz specialised in microbiology as a veterinarian. His doctoral thesis took him to the rainforest of Côte d‘Ivoire in the early 2000s - and straight into zoonosis research. At that time, chimpanzees were dying inexplicably in the Taï National Park. Leendertz wanted to find out why. He was able to show that a new type of anthrax was behind it. The pathogen, a bacterium that infects the skin, lungs or intestines, is highly infectious. Humans can easily become infected just through skin contact with diseased or even dead animals, sometimes with a fatal outcome if antibiotics are not given at an early time. “That was when I realised how important zoonosis research is and that I definitely wanted to continue my research in this area,“ Leendertz recalls. Today he is one of the world‘s leading experts in the field. In 2020, he was awarded the “Champions of the Earth Award”, the United Nations‘ most prestigious environmental prize.
What One Health means
But under what conditions do pathogens make the leap from animals to humans in the first place? And how can it be prevented? These are precisely the questions addressed by the One Health research approach. Based on the triad of healthy environment, healthy animals, healthy humans. “The key point is: We cannot look at human health in isolation. It is closely intertwined with the environment and the animal world, everything is interrelated,“ says Leendertz. “If we preserve a healthy environment, or try to restore it as best we can, we can ensure that animals stay as healthy as possible and protect human health at the same time.“
„The point is: we cannot look at human health in isolation. It is closely linked to the environment and the animal world, everything is interrelated“
Prof. Fabian Leendertz, Founding Director of the HIOH
But any disturbance has an impact on the system as a whole. Intensive land use and climate change throw ecosystems out of balance, leading to changes in the communities of species and weakened wildlife populations - thereby providing ideal conditions for pathogens. “Specifically regions with very high natural biodiversity, such as the tropics, also have a high diversity of microorganisms and that includes pathogens,“ Leendertz explains. “And they can spread quite readily if the biodiversity decreases and individual species multiply massively. Mostly these are so-called synanthropic species, which have been able to adapt well to the landscape changes made by humans.“ In addition, humans are encroaching more and more on wild animal habitats, for example to gain new land for agricultural use or the mining of raw materials. This, in turn, increases the likelihood of human-animal contact along with the risk of transmission. “Human behaviour also plays an important role,“ says Leendertz. “Game hunting has changed a lot in the tropics. It used to be common to hut antelopes, monkeys, pigs or other large game. Since many of these species are locally extinct or severely depleted, people are starting to hunt smaller species as well. For example, larger rodents or fruit bats - and this increases the risk enormously of coming into contact with new, possibly dangerous pathogens.“
Research at the HIOH
One Health calls for a holistic approach, which the scientists at the HIOH want to pursue together with their founding partners: the University of Greifswald, the Greifswald University Medicine and the Friedrich-Loeffler-Institut (Federal Research Institute for Animal Health) - and in close cooperation with their colleagues at the HZI. A wide variety of disciplines will join forces in future research projects: human and veterinary medicine, microbiology, virology, epidemiology, drug research, biodiversity research, ecology, evolutionary biology, anthropology and sociology. “In order to keep the big picture in focus, we have to merge expertise and know-how from a wide variety of fields,“ says Leendertz. “This is an exciting challenge that I am very much looking forward to.“
In a large-scale One Health long-term observational study, the HIOH researchers will take a close look at two different model regions, in each of which intensive agriculture and hunting play an important role. One study area is located in the African tropics, the other in Mecklenburg-Western Pomerania. In both regions, they want to study, among other aspects, climatic conditions, biodiversity, wildlife populations as well as microorganisms present, and set up a cohort from the local population, which will include farmers and hunters in particular. “My most important goal here is to make sure we design and establish the observational study in such a well thought-out way that it still produces useful data 30 or 40 years from now and we get good results during this time,“ says Leendertz. “I will long be retired by then, but I already can’t wait to see the One Health long-term results, because they are unprecedented.“
Fabian Leendertz worked at the Robert Koch Institute (RKI) for 22 years. Working with six other cooperation partners, he has conducted research since 2020 on the so-called BIODIVAFREID project, which is coordinated by the University of Antwerp. Leendertz is continuing this three-year research project at the HIOH. Dr Lorenzo Lagostina, a former RKI scientist now at the HIOH, explains what their research is all about: “We aim to find out how changes in biodiversity in African forests affect the communities and health of potential vectors – primarily small rodents or bats. In addition to recording the biodiversity of the small mammal populations using molecular methods, we are testing the animals for various pathogens. The main focus here is on the pathogens of Ebola, Corona and monkeypox.”
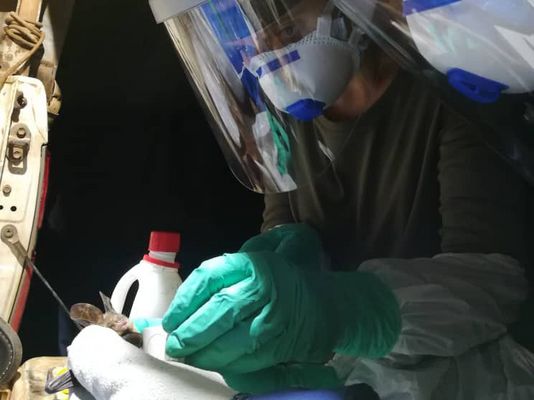
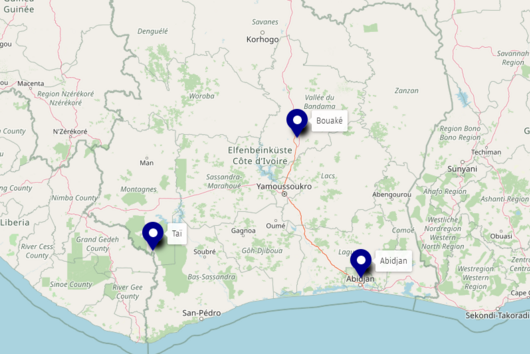
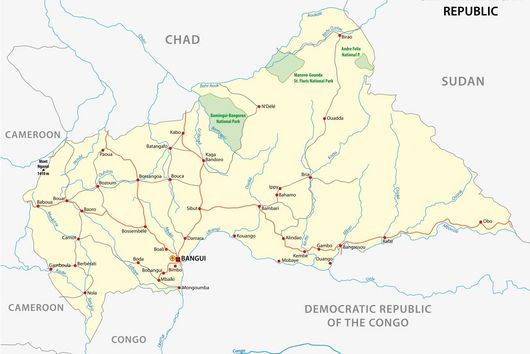
To obtain sample material, the scientists have systematically set up traps in parallel in two different study areas, one in the Democratic Republic of Congo and one in Côte d‘Ivoire. “There are traps within a village, also in houses, at the edge of the settlement, as well as five and eight kilometres away, in the latter case within a national park,“ says Lagostina. “Using this gradient, we can map the human impact on the different habitats of the animals and correlate it to the respective presence of pathogens.“ Swabs are taken from the animals‘ mouth and anus and a few drops of blood are drawn before they are released. “We are working very closely with our partners in both African countries. We want to continue this in future projects and strengthen applied research and surveillance on site,“ adds Leendertz. The samples are then tested in detail by the HIOH research group both in the African partner laboratories and in Greifswald using various molecular diagnostic methods. “We search for viral RNA or DNA of potential candidate pathogens,“ says Lagostina. “Beyond distinguishing whether they are Ebola or monkeypox viruses, we can also identify different variants of the individual viruses and determine their distribution over the different study areas.“
How One Health can help
The major goal pursued by One Health is prevention and preparedness for future pandemics. For that, we need to know exactly where the dangers lie. Which pathogens are we dealing with? How do they change over time? Which animals are potential vectors? Where and how can transmissions take place? “We want to get to the bottom of these questions so that reasonable preventive measures can be taken and implemented efficiently,“ says Leendertz. This includes environmental protection, establishing food security, improving medical care, educating the local population on where possible risks of infection lie and advising them on how to manage them. “At the same time, of course, we have to prepare for an emergency: constantly monitoring dangerous candidate pathogens, tracking down as-of-yet unknown pathogens, advancing vaccine and drug research and addressing the issue of resistance. All this is also part of One Health,“ says Leendertz.
The environment, animals and humans always resonate together, just like the notes in a triad. Fabian Leendertz and his HIOH team are propelling One Health research at full tilt to identify dissonances as early as possible and resolve them optimally - for healthy coexistence of the environment, animals and humans.









![[Translate to English:] Glaskugel mit Schema zum One Health Ansatz](/fileadmin/HIOH/__processed__/6/3/csm_header_6000px-format4-3-150dpi_72_f8c5bcd651.png)
![[Translate to English:] Portrait von Prof. Fabian Leendertz](/fileadmin/HIOH/__processed__/6/5/csm_Portrait-Fabian-Leendertz-Copyright_Stefan_Sauer_72_bf4b2ab673.jpg)
![[Translate to English:] Portrait von Fabian Leendertz © Stefan Sauer](/fileadmin/HIOH/__processed__/8/0/csm_copyright_Stefan_Sauer_72_1396ecab68.jpg)
![[Translate to English:] Hauptgebäude der Universität Greifswald](/fileadmin/HIOH/__processed__/b/9/csm_copyright_Greifswald_University_Jan_Messerschmidt_c6f86932c5.jpg)